No products in the cart.
Categories
My Publication
Shopping cart (0)
Subtotal: ₹0.00
Spend ₹2,000.00 to get free shipping
Congratulations! You've got free shipping.
₹375.00 Original price was: ₹375.00.₹360.00Current price is: ₹360.00.
Authors: Dr. D.K. Chaubey
ISBN: 978-81-922053-9-7.
Publication: The Krishna Publishing House, Gorakhpur
Pages: 406 Pages
Class Label: B.Sc. 1st Semeste
10 Students are viewing this books right now
🚚 Shipping Rates & Delivery Dates: See at Checkout!
शिपिंग दरें और डिलीवरी की तारीखें: चेकआउट पर देखें!
🎉 Special Offers on Books: Explore at Checkout!
📚 किताबों पर विशेष ऑफर: चेकआउट पर देखें!
💳 Pay Prepaid & Get Extra 5% Off!
प्रीपेड भुगतान करें और अतिरिक्त 5% छूट पाएं!
🛒 Shop All Books Now!
🛒 सभी किताबें अभी खरीदें!
🚚 Shipping Rates & Delivery Dates: See at Checkout!
शिपिंग दरें और डिलीवरी की तारीखें: चेकआउट पर देखें!
🎉 Special Offers on Books: Explore at Checkout!
📚 किताबों पर विशेष ऑफर: चेकआउट पर देखें!
💳 Pay Prepaid & Get Extra 5% Off!
प्रीपेड भुगतान करें और अतिरिक्त 5% छूट पाएं!
🛒 Shop All Books Now!
🛒 सभी किताबें अभी खरीदें!
Your Payment is 100% Secure
| Weight | 0.500 kg |
|---|---|
| Dimensions | 18 × 2 × 24 cm |
| Types Of Book | Paperback |
| Publication | The Krishna Pub. Gorakhpur. |
| Writer | Dr. D.K. Chaubey (L.B.S.S.P.G. College, Anand Nagar, Mahrajganj, Up). |
| Subject Of Book | Chemistry |
| Class Of Book | B.Sc. 1st Year 1st Sem. |
| No. Of Pages | 406 Pg |
| Language Of Book | English |
Authors: Dr. D.K. Chaubey (L.B.S.S.P.G. College, Anand Nagar, Mahrajganj, UP),
. Prof. Sudha Yadav (D.D.U. Gorakhpur University, Gorakhpur),
.Prof. U.N. Tripathi (D.D.U. Gorakhpur University, Gorakhpur, UP),
.Dr. Surabhi Chaubey (M.M.M. Technical University, Gorakhpur)
ISBN: 978-81-922053-9-7. Publication: The Krishna Publishing House, Gorakhpur
Pages: 406 Pages
Class Label: B.Sc. 1st Semeste
This comprehensive textbook is designed for B.Sc. 1st Semester students, offering a detailed exploration of fundamental chemistry concepts and applications. It integrates theoretical knowledge with practical exercises to support a deep understanding of chemistry principles.
Contents:
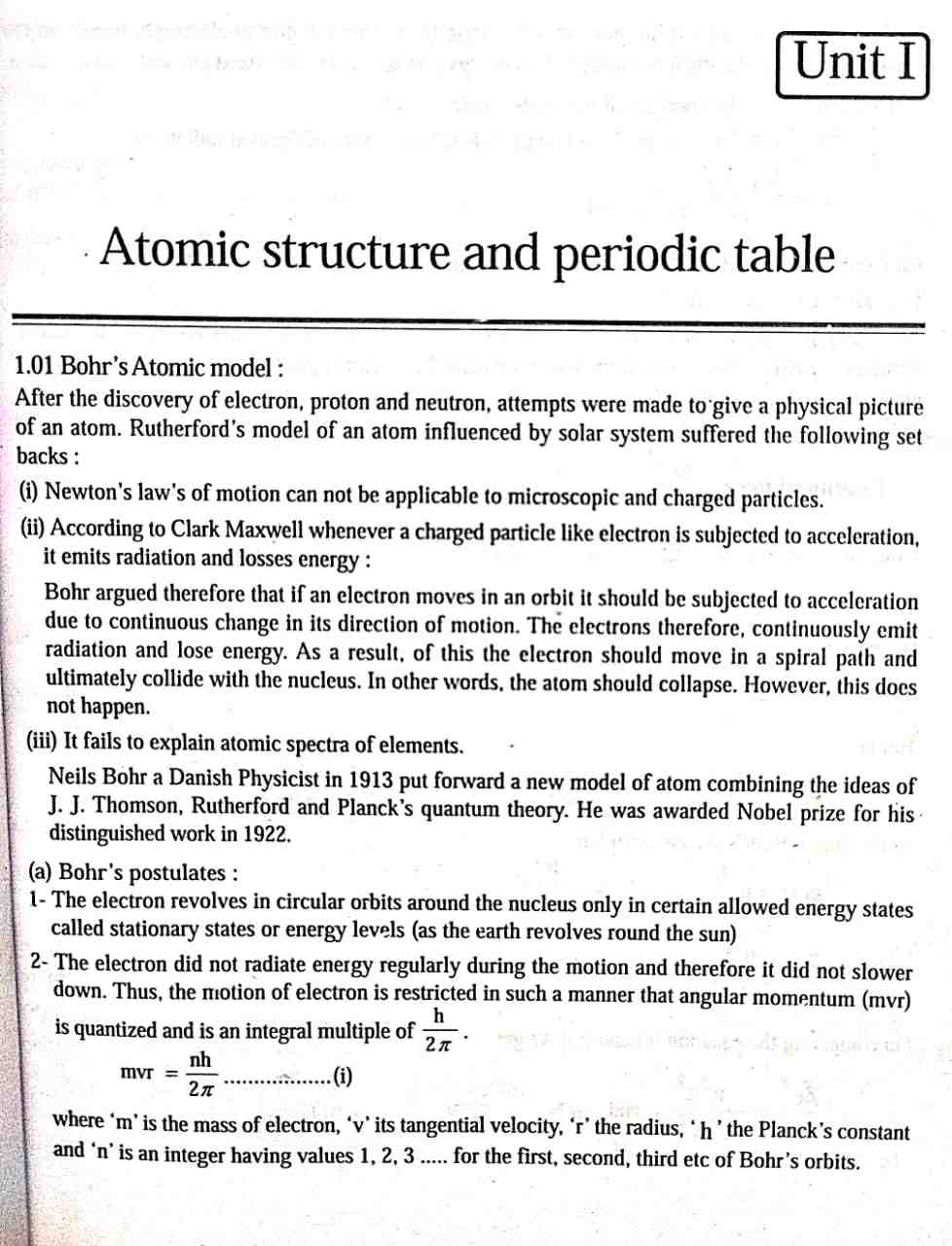
Unit 1: Atomic Structure and Periodic Table
1. Bohr’s Atomic Model
2. Wave Mechanical Concept of Atoms (de Broglie Hypothesis)
3. Heisenberg’s Uncertainty Principle
4. Schrodinger’s Wave Equation
5. Shapes of Orbitals
6. Quantum Numbers
7. Hund’s Rule of Maximum Multiplicity
8. Pauli’s Exclusion Principle
9. Energy Level Diagram of Atom
10. Aufbau’s Principle
11. Periodic Table
12. Electronic Configurations of Elements from Atomic Number
13. Screening Effect or Shielding Effect
14. Periodic Table and Their Characteristics
15. Problems with Solutions
16. Exercise
17. Multiple Choice Questions
18. Answer
Unit 2: Chemical Bonding (Molecular Polarity, Weak Chemical Forces & Simple Bonding Theories of Molecules)
1. Ionic or Electrovalent Bond
2. Variable Electrovalency
3. Inert Pair Effect
4. Lattice Energy
5. Ion Deformation
6. Fajan’s Rules
7. Factors Affecting the Polarizability of Anions
8. Significance of the Concept of Polarization
9. Covalent Bond
10. Polar and Non-Polar Covalent Bonds
11. Octet Rule
12. Variable Covalency
13. Coordinate Bond
14. Odd Electron Bond
15. Metallic Bond
16. Hydrogen Bond
17. Sidgwick-Powell Theory
18. Valence-Shell Electron Pair Repulsion Theory
19. Hybridization of Atomic Orbitals
– sp-Hybridization
– sp²-Hybridization
– sp³-Hybridization
– sp³d Hybridization
– sp³d² Hybridization
– sp³d³ Hybridization
– Effect of Lone Pairs of Electrons on Geometry
20. Molecular Orbital Theory
21. Problems with Solutions
22. Exercise
23. Multiple Choice Questions
24. Answer
Unit 3A: Periodic Properties of Atoms
1. Size of Atoms and Ions
2. Periodic Variation of Atomic and Ionic Radii
3. Structure of Ionic Solids
4. Ionization Potential
5. Electron Affinity
6. Electronegativity
7. Points to Remember
8. Typical Problems with Solutions
9. Exercise
10. Multiple Choice Questions
11. Answer
Unit 3B: Acid-Base Concepts
1. Bronsted-Lowry Acid-Base Concept
2. Lewis Concept of Acids and Bases
3. Relative Strength of Acids
4. Relative Strength of Bases
5. Hard Soft Acid-Base (HSAB) Principle
6. Important Points
7. Exercise
8. Multiple Choice Questions
9. Answer
10. Hints
Unit 4: Recapitulation of Basics of Organic Chemistry & Mechanism of Organic Reactions
1. Molecular Structure
2. Bond Orbitals Involved in Bonding
3. Hybridization
4. Bond Parameters
– Bond Lengths
– Bond Energies
– Bond Angles
5. Van der Waals Forces
– Dipole-Dipole Interactions
– Ion-Dipole Interactions
– London Forces
6. Types of Electronic Displacements
– Inductive Effect
– Electromeric Effect
7. Localized and Delocalized Bonds
8. Resonance
9. Hyperconjugation
10. Tautomerism
11. Electrophilic and Nucleophilic Reagents
12. Types of Reagents
13. Breaking and Making of Covalent Bonds
– Free Radicals
– Carbocations
– Carbanions
14. Carbenes
15. Nitrenes
16. Benzyne
17. Inclusion Compounds
18. Organic Reactions and Methods of Determining Their Mechanism
19. Homo-Lumo Interaction
20. Types of Organic Reactions
21. Exercise
22. Multiple Choice Questions
23. Answer
Unit 5: Stereochemistry
1. Stereoisomerism
2. Optical Isomerism
– Lactic Acid
– Tartaric Acid
3. Resolution of Enantiomers
4. Racemic Mixture or Racemic Modification
5. Inversion of Configuration
6. Retention
7. Walden Inversion
8. Representation of 3-Dimensional Molecules
9. Relative Configurations
10. Absolute Configurations
11. Interconversion Between Fischer and 3-Dimensional Wedge Formula
12. Geometrical Isomerism
13. Conformational Isomerism
– Conformations of Ethane
– Conformation of n-Butane
– Conformations of Cycloalkanes
14. Typical Problems with Solutions
15. Exercise
16. Multiple Choice Questions
17. Answers
Unit 6: Basic Knowledge of Computers
1. Introduction
2. Classification of Computers
3. Generation of Computers
4. Personal Computer
5. Main Parts of the System Unit
6. Number System
7. Conversion of Numbers to Decimal System
8. Radix
9. Binary Arithmetic
10. Software
11. Hardware
12. Computer Languages or Programming Languages
13. Terminology Related to Computers
14. Abbreviations Related to Computers
15. Important Shortcut Keys for Computers
16. Conversion Units
17. Multiple Choice Questions
18. Answer
Unit 7: Mathematical Concepts of Chemistry
1. Logarithms
2. Antilogarithms
3. Differentiation
– Problems with Solutions
4. Partial Differentiation
– Problems with Solutions
5. Maxima and Minima
6. Integration
– Definite Integral
– Indefinite Integral
– Problems with Solutions
7. Probability
8. Factorial
9. Linear Graph
10. Exercise
11. Answer
Unit 8: Environmental Chemistry
1. Atmosphere
2. Hydrosphere
3. Lithosphere
4. Biosphere
5. Pollution
6. Greenhouse Effect
7. Acid Rain
8. Ozone Layer Depletion
9. Points to Remember
10. Exercise
11. Multiple Choice Questions
12. Answer
Be the first to review “Fundamental Chemistry 1St Sem. DKC” Cancel reply
Related products
Sale
Fundamentals Of Chemistry 5th Sem.Paper 1st Pragati
🔥 3 items sold in last 7 days
Rated 5.00 out of 5
Sale
Chemistry-5th Sem. Rearrangements &Chemistry Of Group Elements Paper-2nd Pragati
🔥 2 items sold in last 7 days
Sale
Quantum Mechanics & Spectroscopy B.Sc. 5th Sem Testbook Kanha
🔥 7 items sold in last 7 days
Sale
Algebra B.Sc. 2nd Year 3rd Sem.Paper 1st(Part-A) Vandana
🔥 6 items sold in last 7 days
Sale
Solid State and Nuclear Physics (B.Sc. Sem-VI)
🔥 4 items sold in last 7 days
You may add any content here from XStore Control Panel->Sales booster->Request a quote->Ask a question notification
At sem a enim eu vulputate nullam convallis Iaculis vitae odio faucibus adipiscing urna.





























Reviews
There are no reviews yet.