No products in the cart.
Categories
My Publication
Shopping cart (0)
Subtotal: ₹0.00
Spend ₹2,000.00 to get free shipping
Congratulations! You've got free shipping.
₹275.00 Original price was: ₹275.00.₹260.00Current price is: ₹260.00.
Publication: Ram Prasad Publication, Agra
Authors:
- R.C. Saraswat [K.R. (P.G.) College, Mathura]
- Ketan Topiwala [P.M.B. Gujarati Science College, Indore]
- A.K. Tiwari [S.V.M. Science & Technology, P.G. Lalganj, Pratapgarh]
- Vishal Goswami [B.S.A. (P.G.) College, Mathura]
Pages: 296
Edition: 2023-24
ISBN: 978-93-85630-89-7
43 Students are viewing this books right now
🚚 Shipping Rates & Delivery Dates: See at Checkout!
शिपिंग दरें और डिलीवरी की तारीखें: चेकआउट पर देखें!
🎉 Special Offers on Books: Explore at Checkout!
📚 किताबों पर विशेष ऑफर: चेकआउट पर देखें!
💳 Pay Prepaid & Get Extra 5% Off!
प्रीपेड भुगतान करें और अतिरिक्त 5% छूट पाएं!
🛒 Shop All Books Now!
🛒 सभी किताबें अभी खरीदें!
🚚 Shipping Rates & Delivery Dates: See at Checkout!
शिपिंग दरें और डिलीवरी की तारीखें: चेकआउट पर देखें!
🎉 Special Offers on Books: Explore at Checkout!
📚 किताबों पर विशेष ऑफर: चेकआउट पर देखें!
💳 Pay Prepaid & Get Extra 5% Off!
प्रीपेड भुगतान करें और अतिरिक्त 5% छूट पाएं!
🛒 Shop All Books Now!
🛒 सभी किताबें अभी खरीदें!
Your Payment is 100% Secure
| Weight | .350 kg |
|---|---|
| Dimensions | 16 × 1 × 24 cm |
| Types Of Book | Paperback |
| Publication | Ram Prasad Publication, Agra |
| Writer | R.C. Saraswat [K.R. (P.G.) College, Mathura.] |
| Subject Of Book | Chemistry |
| Class Of Book | B.Sc. 3rd Semester Testbook |
| Pages Of Book | 296 |
| Edition Of Book | 2023-24 |
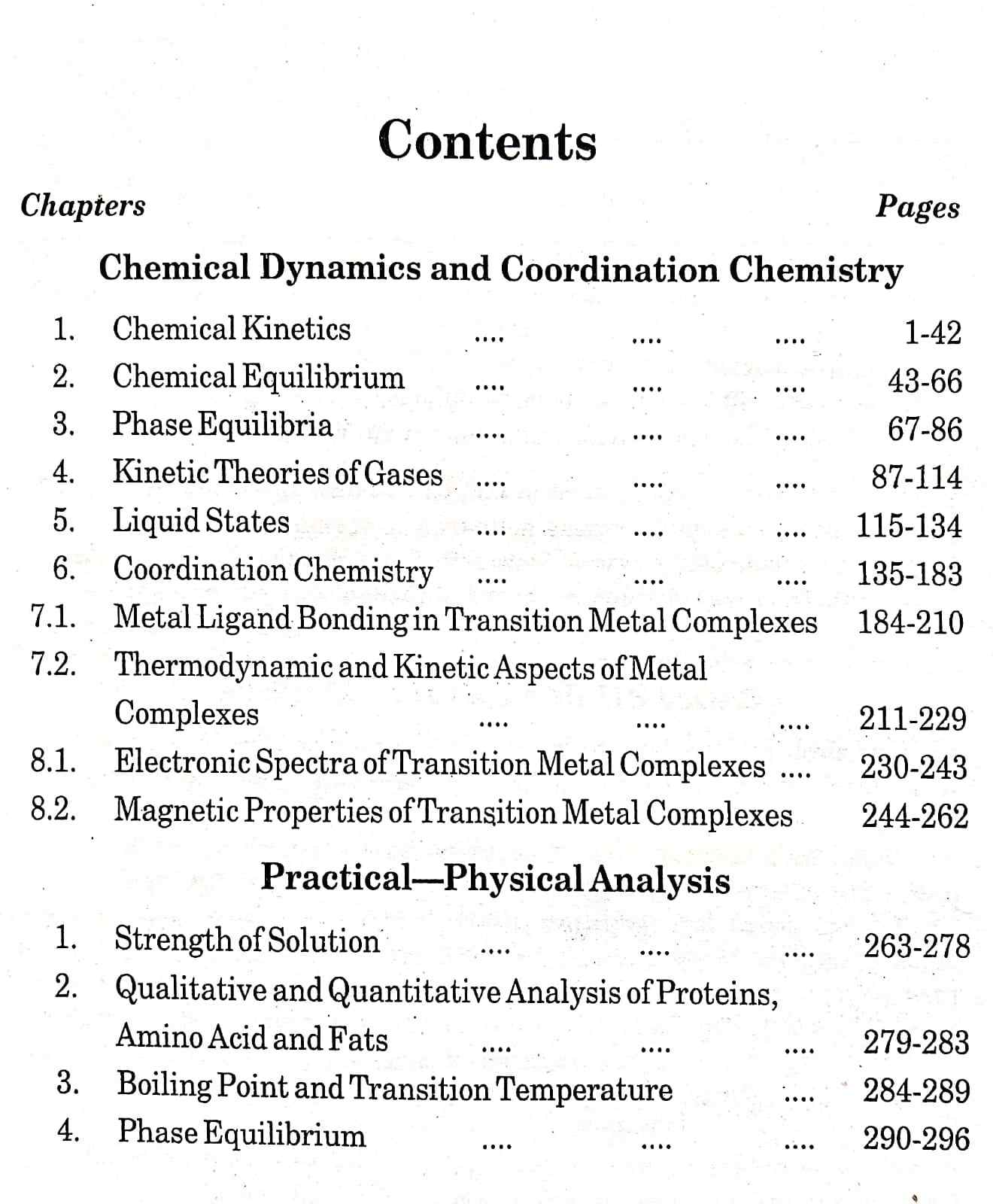
Contents Overview:
Chemical Dynamics and Coordination Chemistry
1. Chemical Kinetics (Pages 1-42)
– Detailed analysis of the rates of chemical reactions
– Factors affecting reaction rates
– Mechanisms of complex reactions
2. Chemical Equilibrium (Pages 43-66)
– Concepts of dynamic equilibrium in chemical systems
– Le Chatelier’s principle and its applications
– Calculations involving equilibrium constants
3. Phase Equilibria (Pages 67-86)
– Phase rule and its applications
– Phase diagrams of single and multiple component systems
– Interpretation of phase transitions
4. **Kinetic Theories of Gases** (Pages 87-114)
– Molecular theory of gases
– Distribution of molecular speeds
– Real gases and deviations from ideal behavior
5. **Liquid States** (Pages 115-134)
– Properties of liquids and their molecular basis
– Viscosity and surface tension
– Theories explaining liquid behavior
6. **Coordination Chemistry** (Pages 135-183)
– Structure and bonding in coordination compounds
– Nomenclature and stereochemistry of complexes
– Stability constants and factors influencing complex stability
7. **Transition Metal Complexes:**
1. **Metal Ligand Bonding in Transition Metal Complexes** (Pages 184-210)
– Crystal field theory and ligand field theory
– Molecular orbital theory as applied to coordination compounds
2. **Thermodynamic and Kinetic Aspects of Metal Complexes** (Pages 211-229)
– Thermodynamic stability of complexes
– Kinetics of complex formation and dissociation
8. **Electronic and Magnetic Properties of Transition Metal Complexes:**
1. **Electronic Spectra of Transition Metal Complexes** (Pages 230-243)
– Electronic transitions and spectroscopic techniques
– Interpretation of spectral data
2. **Magnetic Properties of Transition Metal Complexes** (Pages 244-262)
– Types of magnetic behavior
– Magnetic susceptibility and its measurement
### Practical Physical Analysis
1. **Strength of Solution** (Pages 263-278)
– Methods for determining solution concentration
– Preparation and standardization of solutions
2. **Qualitative and Quantitative Analysis of Proteins, Amino Acids, and Fats** (Pages 279-289)
– Techniques for protein analysis
– Determination of amino acids and fats
3. **Boiling Point and Transition Temperature** (Pages 290-296)
– Measurement techniques
– Practical applications in physical chemistry
4. **Phase Equilibrium** (Pages 290-296)
– Experimental determination of phase equilibria
– Applications in various chemical systems
**Summary:**
This comprehensive textbook covers key concepts in chemical dynamics and coordination chemistry, complemented by practical physical analysis techniques. It is an essential resource for B.Sc. students, providing both theoretical insights and hands-on experimental knowledge. Each chapter is carefully structured to build a solid understanding of complex topics, making it a valuable reference for academic and professional growth in the field of chemistry.
Be the first to review “Chemical Dynamics And Coordination Chemistry With Practical B.Sc. 3rd Sem. Ramprasad” Cancel reply
Related products
Sale
Fundamentals Of Chemistry 5th Sem.Paper 1st Pragati
🔥 3 items sold in last 7 days
Rated 5.00 out of 5
Sale
Physics-5 Quantum Mechanics And Spectroscopy Pragati
🔥 5 items sold in last 7 days
Sale
Mathematics-5 Group And Ring Theory And Linear Algebra Pragati
🔥 7 items sold in last 7 days
Sale
Bharat Me Samajik Parivertan Avn Samajik Andolan B.A. 3rd Sem.Test Book Samajshastra Ramprasad
🔥 7 items sold in last 7 days
Sale
Molecular Biology And Bioinformatics Botany 2nd Paper Ramprasad
🔥 6 items sold in last 7 days
You may add any content here from XStore Control Panel->Sales booster->Request a quote->Ask a question notification
At sem a enim eu vulputate nullam convallis Iaculis vitae odio faucibus adipiscing urna.























Reviews
There are no reviews yet.